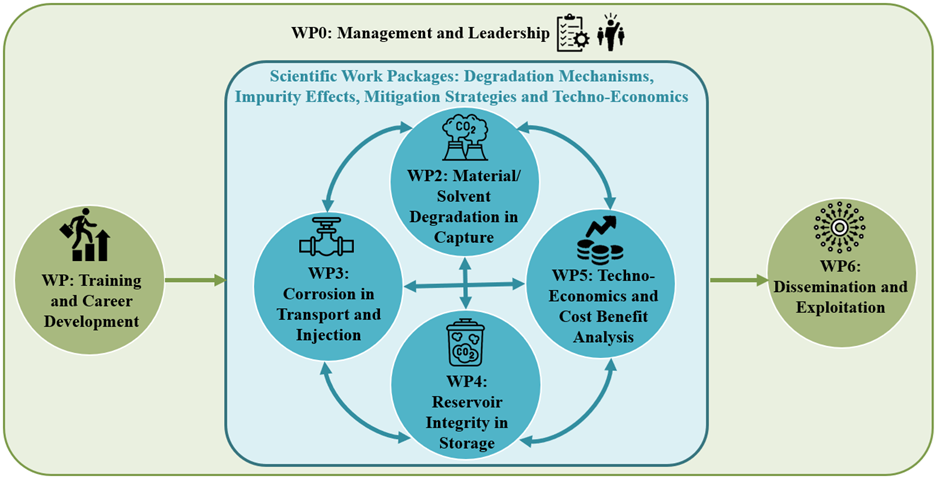
Material/solvent degradation in capture
Despite being the most mature and industrially developed technology for post-combustion CO2 capture, absorption by aqueous amine-based solutions is not without its challenges. In particular, the solvent degrades over time due to high temperature regeneration, as well as contact with feed stream impurities and plant construction materials.
State-of-the-art and remaining challenges: The variety of amine solvents investigated for CO2 capture exhibit different process performance and rates of degradation. Each are expected to exhibit varying sensitivity to impurities, with the most influential suggested to be O2, NOx, SOx and dissolved metals. Degradation pathways are characterised as thermal or oxidative, yet in CO2 capture plants, both these processes occur together. At the laboratory scale, oxidative and thermal degradation are typically studied in isolation, with most tests avoiding temperature cycling due to the complexicity of cost. Such laboratory simplifications have created notable differences in solvent degradation behaviour compared to Pilot facilities.
A large number of degradation products have been identified from the most commonly used and studied amines, such as monoethanolamine (MEA), piperazine (Pz), 2-aminomethyl propanol (AMP), and more. Over the last few decades, a number of analytical techniques have been developed to study degradation of solvent (particularly chromatography methods), shedding new light on the complex degradation pathways. Despite extensive research into solvent degradation, there is still a need to better understand such processes. In the recent ACT ALIGN-CCUS and LAUNCH EU projects, solvent and capture infrastructure degradation as a result of NOx and metallic impurities, were identified are key issues. Despite these findings, limited laboratory studies exist where solvent degradation is considered in the presence of actively corroding materials. As a result, there exist no degradation models for solvents which incorporate the collective effect of impurities and metallic ions, let alone the connection through to corrosivity of the capture plant infrastructure.
In relation to mitigation of solvent and infrastructure degradation, scavengers (e.g. sulphite) and chelating agents (e.g. ethylenediaminetetraacetic acid (EDTA)) have been proposed to reduce oxidative degradation. In addition, corrosion inhibitors such as NaVO3 have been evaluated as regards their ability to prevent infrastructure corrosion. However, the potential implications of solvent degradation inhibitors on infrastructure corrosion, and the ability of these corrosion inhibitors to influence the rate of solvent degradation have received little attention. Finally, it has been suggested that a ‘natural’ corrosion mitigation strategy to reduce infrastructure degradation exists via the formation of protective corrosion products (e.g. FeCO3) on carbon steel in amine solvent. However, the kinetics of forming these protective products and their stability in amine solution chemistries has not been explored in detail.
Innovation: In WP2, and unique to MISSION-CCS, the relationship between capture plant corrosion and solvent degradation will be explored when both processes occur concomitantly in the same system in the presence of impurities. Such work will be supported through controlled studies into both solvent degradation and corrosion to identify degradation pathways and corrosion rates/mechanisms, respectively. Such advanced understanding will be achieved through the implementation of state-of-the-art analytical (e.g. chromatography) and electrochemical techniques (e.g. impedance spectroscopy).
The originality of the proposed work lies not only within the simultaneous laboratory study of both phenomena, but in the development and/or evaluation of new or existing techniques to control solvent degradation and corrosion. In particular, MISSION-CCS will determine how solvent chemistry can be tuned to support the formation of protective corrosion products, providing a novel method to enable prolonged integrity of the capture plant infrastructure; such an approach has not been considered before. Most importantly, the synergistic and/or antagonistic effects of mitigation strategies/chemistries on operational issues will be examined; this is rarely considered, yet is fundamentally important to successful operation. Scalability of mitigation strategies will be tested at Pilot Scale through the development and integration of a series of online electrochemical, infrared and spectroscopic monitoring tools into DTU’s Pilot facility.
Corrosion in transport and injection
Pipeline transportation and injection are critical components linking capture to storage. Researchers have suggested that achieving the targeted deep reductions in CO2 emissions would require an extensive pipeline networks from carbon steel. CO2 pipelines must be constructed and designed optimally, so they are reliable and safe to operate, posing minimal risk to the local population and environment. Carbon steel is, however, susceptible to corrosion in flue gas environments because of the presence of CO2, H2O, O2, SOx, H2S, NOx and other impurities.
State-of-the-art and remaining challenges: Currently, there is no universally agreed specification for the CO2 stream composition to ensure safe transport, nor significant industrial experience of anthropogenic CO2 transmission. A number of specifications and recommendations for maximum impurity concentrations have been published but these mostly fail to consider corrosion, and are defined based on HSE (toxicity limits) and reservoir requirements. None of the recommendations by DYNAMIS, NETL and CarbonNet were intended to be used in actual projects without further refinement, nor were they validated experimentally from a corrosion perspective. The composition proposed for the Northern Light Project was experimentally tested by the Institute for Energy (IFE). Despite this specification being stricter than all others, it was shown that impurities within the system generate sulphuric and nitric acids, indicating the need to better understand pipeline corrosion susceptibility when exposed to impure CO2. Furthermore, for amine solvent capture systems, there is significant potential for the amine (or its degradation products) to be carried over into the pipeline transport system, yet the consequences of this on pipeline corrosion have not been explored.
Research by IFE as part of the ALIGN-CCS EU project, shows that it is not possible to give universal recommendations on stream composition. The maximum tolerable concentration of any single impurity is dependent on the concentration of the other impurities in the CO2 stream, as well as the operating conditions. A further challenge arises from limitations in the ability to simulate realistic conditions at laboratory scale that are translatable to the field. Issues with condensation during depressurisation at the end of experiments, as well as challenges with maintaining impure CO2 composition for prolonged periods have produced misleading results. To our knowledge, there is no system currently able to replicate the hydrodynamics of a CO2 pipeline whilst maintaining impurity levels and there has been little consideration of the localised corrosion that is evident in the presence of impurities.
The majority of pipeline infrastructure for pipeline transportation of impure dense phase CO2 will consist of carbon steel. However, the potential exposure of downhole materials to water-saturated impure CO2 and/or even impure CO2-containing water/brine may well necessitate the use of more corrosion resistant alloys (CRAs). However, the potential formation of nitric and/or sulphuric acid has the potential to de-passivate CRAs, resulting in excessive corrosion of such expensive alternatives. To date, studies in such environments are limited, yet those that do exist suggest that these environments provide a significant threat to passive materials.
Innovation: In light of recent research findings, MISSION-CCS will not focus on the identification of a universal CO2 stream for safe transportation. Instead, considering the reported challenges with replicating CO2 transport at laboratory scale, our attention is directed towards developing a unique laboratory system with associated monitoring techniques to reliably predict corrosion rates anticipated in the field under both dense phase CO2 and aqueous environments. This will provide a system capable of making more informed decisions with regard to impurity control and/or material selection. The researchers in WP3 will utilise this system, along with more conventional experimental methods, to develop a more comprehensive understanding of the degradation mechanisms in the presence of impurities. The foci will be on statistical localised corrosion assessment in the presence of impurities, as well the implications of amine carry-over into the transportation network (both unique to MISSION-CCS). WP3 will examine CRA passive film stability in such environments as a means of improving material selection processes. Specific novelty in this area will involve consideration of newly developed alloys capable of bridging the gap between austenitic stainless steels and nickel alloys for extremely corrosive environments e.g. Sanicro 35.
Reservoir integrity in storage
Impurities in the CO2 stream, such as amines, SOx, and NOx are polar and chemically active species. They can impact on the integrity of the reservoir as impurities, after interacting with the in-situ fluids, will partition in the supercritical CO2 and aqueous phases, and any residual oil phase (if the storage site is a depleted hydrocarbon reservoir). Such interactions can induce changes in (a) the porosity and permeability of the “host”, affecting the reservoirs’ injectivity and storage potential, (b) the formation strength and ability to retain CO2 over time. Three main areas within the reservoir need to be considered: (Area I) the reservoir rock i.e. formation itself, (Area II) the sealant (cap rock and faults) that ensures retention of the CO2 phase within the reservoir, and (Area III) well-bore area including cement-rock-steel interfaces where the fresh CO2 stream comes into contact with the reservoir rock or steel pipe. Recently, Area III had been identified as a critical location where leak paths are likely to develop.
State of the art and remaining challenges: In-depth understanding of the effects of impurities on reservoir integrity is lacking due the complex interplay involving multiphase flow, phase equilibrium, and chemical reactions, as well as the influence of the rock type and injection conditions. While in the past decades many studies considered the long term integrity of geological formations in the context of the reservoir rock in general and the seals in particular (cap rock and faults), the role of impurities (e.g. amines, SOx, and NOx) on a formation’s and seal’s capacity to store and retain an injected CO2 phase has received much less attention.
However, in view of the practicalities of CO2 sequestration and decision making in terms of necessary CO2 phase purity, such detailed knowledge of the effect of impurities on geochemical reactions, mineral transformations, alteration of surface wettability, and reduction of capillary pressure and mechanical integrity of a geological storage is urgently needed. Such knowledge in relation to formation rock is particularly important for establishing the long-term capacity of the reservoir. Research on seals has been largely focussed on the effect of brines, often with little consideration of impurities. The most common cap rock and seal is associated with the presence of a clay rich layer. In such rocks, clay hydration/swelling, dehydration, and shrinkage are of major importance, resulting in decreases and increases in permeability, respectively. The details for these processes may be influenced by the presence of impurities. In addition, fractures may form due to local stress changes, and the mineral precipitation processes, which may close them, can be highly influenced by impurities and the detailed impurity related chemistry of the CO2 phase.
Recent studies have highlighted that a potential point of “failure” is related to the carbonation reactions associated with casing cement as well as the external integrity of the casing itself. There are suggestions that Portland cement has the ability to ‘self-heal’ under sc-CO2 conditions, strengthening it and reducing permeability. However, the US Department of Energy highlights that the ratio of solid (cement)-to-solution in laboratory experiments has a noticeable effect on cement carbonation and ultimately whether CaCO3 precipitation occurs. The detrimental effects of carbonation reactions could be more severe when acid impurities (SOx or NOx) are considered, reducing the likelihood of CaCO3 precipitating and ‘healing’. Recent studies have suggested alkali-activated materials have comparable resistance to carbonation. However, the response of both materials to supercritical CO2 has received far less attention, with the effect of acidic impurities ignored completely. When sc-CO2 saturated brine diffuses to the outside of the casing, corrosion will initiate at the steel-cement interface. The production of Fe2+ there is likely to result in the precipitation of mineral deposits at the steel-cement interface, and in the pores of the cement matrix itself, reducing the cement’s permeability. However, due to the Pilling-Bedworth ratio of some corrosion products, their growth may cause cracking of the cement itself. While limited studies have focused on corrosion mechanisms of steel embedded in cement, the contribution of steel dissolution to the cement integrity has been overlooked.
Innovation: WP4 of MISSION-CSS will focus on the two key issues related to CCS reservoir integrity currently overlooked in the literature: (i) Role of impurities on the formation, seal and injection point bulk characteristics through time and space, and (ii) Role of impurities on the evolution of reactions and growth at interfaces (i.e. formation-cement and cement-casing interfaces). An integration of novel experimental work, advanced analytical methods and numerical simulations will allow us to evaluate the interactions between impure supercritical CO2-containing (both water-saturated and aqueous phases) on the reactions with reservoir formations, seals, cement and casing materials through time and space. Only such an integrative approach will provide the understanding of the evolution of interfaces within differing structures in the presence of impurities; knowledge necessary to predict their influence on reservoir long-term capacity and integrity. Furthermore, given the growing interest in the field of low carbon cements, and the potential advantages associated with alkali-activated binders, MISSION-CCS will explore the potential of these novel cement chemistries to retain integrity in impure-CO2 environments.
Techno-economics and cost benefit analysis
The techno-economics (TE) and lifecycle emissions of carbon capture and storage technologies have been extensively studied over the previous 10 years. As a result, the community now has a good understanding of the policy and economic frameworks essential to underpin future large-scale CCS deployments. The strength and weaknesses of a variety of capture approaches have also been extensively explored. However to date, there are limited large-scale CCS deployment projects in progress. As a result, much less attention has been given to analysing uncertainties in the performance of engineering components in the CCS capture, transport and storage chain. Improved understanding of their impact on system performance at the ‘plant-scale’ is essential for future plant design optimisation. This integrating work-package will draw on findings from other WPs to investigate how three key areas of uncertainty (specifically: solvent degradation, corrosion and reservoir degradation) impact on the TE and life cycle performance of future CCS plants.
State of the art and remaining challenges: Improving technology readiness levels (TRLs) mean there is now increasing certainty over the technologies and configurations that future CCS power plant systems will adopt. Thanks to the interconnected nature of the individual processes involved, extensive design optimisation is essential. Key plant-level criteria are the cost of capture per tonne of CO2, and the net CO2 release per unit of electricity delivered. Both criteria should be minimised over the lifecycle of individual plant by taking a systems-level perspective. A particular challenge arises from interactions between downstream and upstream processes with decisions made at the storage reservoir influencing optimal choices at the power plant, as well as conditions in the power plant impacting what is feasible downstream. Choices made to minimise corrosion, for example, may have negative impacts on solvent and reservoir degradation. As a result successful optimisation of CCS systems will require TE and life-cycle emissions models that can take account of these interactions.
As noted previously, the direct impact of certain contaminants on aspects of individual processes in the CCS chain has been considered. However whole classes of contaminants have been neglected, and there has been little attempt to consider upstream/downstream interactions arising from choices made to optimise individual processes. The CO2QUEST project attempted to examine downstream impacts, but took a largely qualitative approach aiming to identify key influencing factors (e.g. toxicity, acidity, corrosiveness). For CCS system optimisation purposes, more robust quantitative TE and lifecycle costing assessment (LCA) relationships will be required.
Innovation: WP5 will quantitatively examine how trade-offs arising from contaminant clean-up/removal at different stages in the CCS chain influence TE and lifecycle carbon emissions associated with future CCS systems suitable for deployment at scale. The focus will be on integrating results from other WPs in the project (i.e. contaminant impacts solvent degradation, corrosion and reservoir degradation) into TE and LCA models, using them to evaluate the whole system effects of different amelioration strategies at each stage, for several prototypical future CCS plant that might be deployed in Europe (most likely Coal-fired, Gas-fired, Bioenergy with carbon capture and storage). MISSION-CCS will model the upstream and downstream interactions at each stage in the CCS chain.
Key questions to address for each prototypical CCS plant include:
· To what extent is the cost of removing key contaminant species at different stages in the CCS chain offset by operational benefits (e.g. improved pipeline and reservoir integrity) and reduced solvent replacement costs?
· What combinations of operational conditions (to avoid contaminant formation) and clean-up approaches maximise the net carbon avoided on a life cycle basis?
· How might different fuel choices influence the results; this is a particular concern for BECCS where a wide range of feedstocks could potentially be used, each having significantly different contaminant make-up?
· What impacts would measures to reduce the constructions emissions from CCS plant (e.g. low carbon cement) have on the operational emissions, and what is the size of any net benefit?